Written by: Richard Bothell and Justin Bothell, Atlas Technologies; Glen Tisdale, Judith Offerle, UHV Aluminum Company
Achieving sub-micron feature sizes on a production basis requires an understanding of the complete processing environment including the vacuum reactor itself. Molecular level chemical contamination demands that all processes factor in the vacuum environment. The vacuum system base pressures are a critical parameter in determining the environmental contribution to wafer contamination. Actual processing levels may be decades above the base pressure, but the base pressure establishes the environmental noise of the system.
Ultra high vacuum (UHV) base pressures reduce the contamination contribution of the vacuum environment. For example, at high vacuum levels (~10 -9 Torr), mono-layer formation takes hours. In order to control molecular level contamination of sub-micron features, UHV base pressure environments must be considered.
One concern with producing UHV environments in aluminum vacuum systems is the cost of the massive pumping required to overcome o-ring seal leaks and vacuum surface outgassing. And to consider stainless steel UHV systems means that cycle times will be increased due to bake out and pump down times. Stainless is a notorious water sponge at these levels and iron and chromium contamination is an issue as well. In addition, stainless systems are heavy and require a large footprint of valuable clean room real estate.
Aluminum UHV Systems
Aluminum vacuum systems have been used in semiconductor production since the beginning. Its’ low cost of fabrication and overall cost of ownership are well understood. Aluminum has become the dominate vacuum system material for many low and high vacuum cluster tools and other processing reactors.
In many cases, the technology required to process aluminum into a UHV system has not been well understood. However, developments in physics laboratories worldwide made it possible. The methods, process, and components used to build large UHV systems for particle accelerators and synchrotrons are now easily adapted for semiconductor processing applications.
Three Technical Advances that Allow Ultra High Vacuum Performance with Aluminum Systems
There are three primary technical challenges that have been met in this endeavor. They are the development of aluminum surface treatment schemes and outgassing reduction, the development of ultrahigh-vacuum-capable aluminum welds, and finally the development of robust metal sealed aluminum flanges. To benefit from the many superior physical properties of aluminum for UHV systems, relatively minor changes in existing manufacturing processes and components are required. Current designs of aluminum process tools can be upgraded to improve vacuum performance. In particular this means improving the outgassing and permeation performance of these systems.
Aluminum Surface Treatment and Outgassing Reduction
Like untreated or machined stainless steel, the off-the-shelf mill grade surface of aluminum must be stripped and prepared for UHV application. Extruded and rolled plate stock cannot be used directly as it tends to be covered with a porous oxide that is relatively thick, typically 150 Å -200 Å. One of the primary reasons for this is that when aluminum is extruded, alloy impurities segregate to the surface where they combine to produce oxide impurities. Further, when aluminum is machined thick oxides can quickly form on the raw machined surface. These surfaces must be properly treated to obtain the thin dense uniform oxide needed to attain UHV. The left half of Figure 1 illustrates the morphology of a typical untreated aluminum surface.
Aluminum Surface Morphologies
For making aluminum UHV compatible, what is required is some means for ridding the surface of any contaminating constituents and then producing a surface oxide that is thin and non-porous.
Ishimaru, of KEK Japan, was the first to apply a concentrated effort toward solving these problems in his efforts to build aluminum vacuum systems for particle accelerators. He employed two primary processing techniques, one for extrusions and the other for machined components. For extruded surfaces Ishimaru would extrude aluminum into an argon-oxygen atmosphere. For machined components he used ethanol as a machining fluid. Both of these techniques enable the aluminum to form a thin native oxide which is dense and non-porous.
These techniques have since been refined to simplify their implementation. Through work at a number of particle accelerator facilities it has become apparent that surface cleaning with simple alkaline solutions is as effective as argon oxygen extrusion and ethanol machining. Alkaline solution cleaning will remove impurity oxides as well as the top layer of aluminum oxide. Any contaminants introduced from machining and that have been accumulated in the aluminum oxide layer will be removed when the aluminum oxide layer is removed. Additionally, when the top layer of the aluminum oxide is removed by these chemicals a thin, dense and non-porous native oxide will form on the newly exposed aluminum surface.
Aluminum Vacuum Flanging
Aluminum high vacuum systems have typically been sealed with elastomeric o-rings. Elastomers both outgas and are permeable to atmospheric gases. Permeation occurs because atmospheric gases are able to dissolve into the elastomer and then diffuse through the material where they are released into the vacuum chamber. Unlike outgassing, permeation cannot be eliminated by baking the system. Eliminating o-rings from the system can significantly reduce pumping requirements, and cost.
Furthermore, research has been conducted to understand the influence of common vacuum impurities on aluminum sputtered films. One study observed the impact of nitrogen contamination generating from atmospheric permeation through O-ring seals. The upshot of this particular study is that nitrogen will readily incorporate itself into an aluminum film while the film is being grown and this nitrogen impurity will noticeably impact the electromigration resistance.
There are two methods for eliminating o-ring gas permeation, both of which support aluminum ultrahigh vacuum systems. The first is an old solution and involves using differentially pumped seals.
Differentially Pumped Sealing Geometry
In a differentially pumped sealing geometry, the main vacuum chamber is isolated from the atmosphere by a secondary evacuated channel which is separately pumped. Since permeation is proportional to the pressure differential, even a rough vacuum in the pump channel can reduce the permeation by several orders of magnitude. The drawback to this sort of scheme is that it requires an auxiliary channel for each vacuum flange and a pump to evacuate these channels. However, for ports that require quick access such as sputter target ports differential pump schemes do provide a solution that can provide the required vacuum performance.
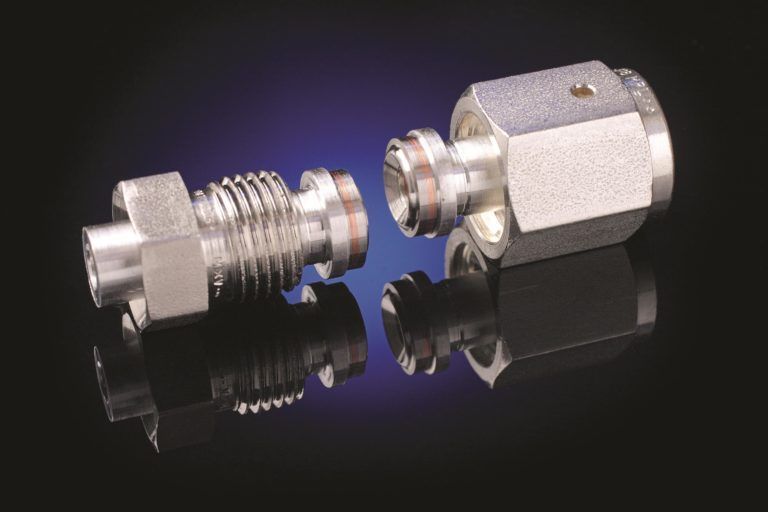
The other solution is to replace the elastomeric seals with metal sealed flanges. The early metal sealed flanges were composed entirely of aluminum and had a ConFlat style knife edge which could seal an aluminum gasket. Typically, the knife edges of these flanges are coated with titanium nitride to strengthen the knife edge. These flanges were subject to three important limitations. First the knife edge, since it is composed of aluminum, is readily damaged because of the softness of the underlying aluminum. Second, overheating the flanges during weld up or while heat cycling, anneals the aluminum, making the knife edge more subject to failure. The third issue is more subtle. Because most vacuum components are stainless steel a typical aluminum flange will serve as a mount for a stainless component. These types of joints do not tolerate temperature cycling well because the differential thermal expansion between the aluminum and stainless steel will loosen the knife edge seal to the gasket and cause the seal to leak. The standard solution for this type of problem has been to provide a temperature differential during system bakeout between the two materials so that the thermal expansions are matched.
A creative solution to this problem was developed by Atlas Technologies and involves the construction of a bi-metal flange. This flange consists of a stainless-steel surface which contains a ConFlat knife edge bonded to an aluminum nipple used for weld up.
Bimetal Flange Detail
The bimetal flange provides a robust sealing surface which is composed of stainless steel, yet it may be readily welded to an aluminum vacuum system. Because the stainless and aluminum surfaces meet at the bond rather than at the gasket, the sealing surfaces are not subjected to differential thermal expansion and consequent leaking. These bimetal flanges serve as ready replacements for elastomeric seals in flanges that do not need to be routinely opened.
Aluminum Welding
The aluminum surface of a vacuum system can be treated so that the outgassing is minimized and the system sealed with flanges to minimize permeation. However, the flanges must still be joined to the system in a fashion that allows for the system to achieve superior vacuum performance. In the case of aluminum vacuum hardware this implies that the flanges must be welded to the remainder of the system with welds that are non-porous so as not to create virtual leaks, and mechanically strong to maintain the structural integrity of the system.
Producing non-porous welds in aluminum is difficult for a number of reasons. First, at high temperatures, hydrogen becomes highly soluble into molten aluminum. When the aluminum subsequently cools, the hydrogen bubbles through the melt and forms pores in the material. Second, aluminum oxide has a high melting temperature which implies that any aluminum oxide that is present at the weld surface will collect within the weld and form a defect layer. Third, because aluminum has a high thermal conductivity, the heat of the weld will tend to be dissipated within the material bulk. And finally, because aluminum shrinks when it solidifies, aluminum welds will be more subject to cracking than their counterparts in steel.
Presence of the effects above does not imply that aluminum welds cannot be produced, it does however mean that producing them will require adequate material preparation and tight control over the processing parameters to insure repeatability. To meet the challenges of welding aluminum, the vacuum community has developed automated TIG welding processes, which when coupled with careful material preparation, yield reliable ultrahigh vacuum welds.
Aluminum as a Vacuum Chamber Construction Material
When learning how to use aluminum for UHV, a vacuum system engineer experienced in stainless steel UHV system designs will be first skeptical, then interested, and then excited by the powerful capabilities that the properties of aluminum offer. Here we shall discuss those properties of aluminum that are of interest to vacuum designers, in our description of these properties we use stainless steel as a convenient benchmark.
Alloys
Aluminum comes in a wide range of alloys which vary as to characteristics. Wrought aluminum alloys are suitable for vacuum chamber fabrication and are designated by a four-digit number which is followed by a temper specification. The first digit specifies the primary alloy composite as indicated in Table 1.
| > 99.00 % Aluminum | 1XXX |
|---|---|
| Copper | 2XXX |
| Manganese | 3XXX |
| Silicon | 4XXX |
| Magnesium | 5XXX |
| Magnesium and Silicon | 6XXX |
| Zinc | 7XXX |
Table 1: Aluminum Alloy Designations
All of the wrought alloys have been used for vacuum chamber fabrication except for the 7000 series which has zinc which has a high vapor pressure at low temperature. The 2000 series alloys are highly weldable. The 6000 series alloys, particularly 6061, and 6063, are commonly and successfully used for ultrahigh vacuum systems.
Machinability
One of the overriding impetuses for fabricating vacuum systems from aluminum is that it is inherently more machinable than materials, such as stainless steel, which have been traditionally used for vacuum component fabrication. The machining cost for 300 series stainless steel is up to 5.5 times that of aluminum.
As indicated previously this ease of machinability is the reason that aluminum has become the material of choice for fabricating semiconductor equipment. Cluster tool components are machined from a single plate of aluminum. These tools are exceptionally rigid, and have a minimal vacuum surface area and occupy a minimal floor space considering their throughput.
Thermal Conductivity
Aluminum has a thermal conductivity that ranges between 170 watt / m K and 230 watt / m K depending on the alloy. Stainless steels by contrast have thermal conductivity’s that are between 14 watt / m K and 16 watt / m K.
High thermal conductivity is an advantage when designing systems that require temperature cycling. This is the case for systems that must be baked to achieve ultrahigh vacuum. An aluminum chamber may be baked and then cooled much more rapidly in development of ultrahigh vacuum than a stainless chamber. And its’ conductivity allows for a complete bake out without the problems of re-condensation of gasses on cool spots common in stainless systems.
Weight
Aluminum is 1/3 the weight of stainless steel. The cost burden associated with weight starts with raw materials handling and progresses through out the manufacturing process including shipping, installation and even architectural engineering and construction.
Magnetic Properties
Aluminum is not magnetic whereas stainless steel, being essentially an alloy of iron exhibits residual magnetism. For applications that involve charged particle beams, the absence of magnetic properties in aluminum is of advantage because it implies that the vacuum system will not modify the fields from the beam control magnets.
Radioactivity
Aluminum in comparison to stainless steel has a much more rapid decay of induced radioactivity. Generally, if both types of materials are bombarded with the same flux of charged particles, the residual radioactivity will be between one and two orders of magnitude less for an aluminum sample than for an identical sample of stainless steel. The nuclear half-life of elements that make up stainless suggests that a particle contamination is always present in stainless steel and a possible source of circuit damage.
Corrosion Properties
The corrosion of both aluminum and stainless steel alloys in reactive gases is a complicated process. However much experimental work has been performed on various alloys in different reactive gaseous environments. The general thrust of these results is that both aluminum and stainless steel are subject to attack by reactive gases, that halogen containing species are typically the most damaging, and that the corrosion activity of any given compound is usually no worse than that of its halogen component alone.
Importantly aluminum is not a worse corroder but simply one with different reaction dynamics.
Outgassing Properties
Vacuum surface gassing is a primary source of process contamination. Water vapor is the most significant contaminate, placing heavy demands vacuum pumping. Metal contamination is a major yield-limiting factor for silicon IC production and iron is one of the most significant contaminates.
The required level of outgassing has not always been achievable with aluminum. Only recently have techniques been developed allow these levels of performance to be achieved repeatably. This improvement in outgassing performance has been one of the principal breakthroughs that has allowed aluminum to become a competent material for the construction of ultrahigh vacuum systems.
Conclusions
Through years of research and improvement, aluminum vacuum systems have become the ideal choice for UHV wafer processing and for base pressure system conditioning. Aluminum ultrahigh vacuum systems are integrated into many leading-edge semiconductor processing systems.
Acknowledgments
We would like to pay our respects to Dr. Hajime Ishimaru deceased. Dr. Ishimaru pioneered the use of aluminum for UHV. We are all forever in his debt The authors would also like to acknowledge James Garner of SMC Corporation for many helpful discussions. Additionally, we would like to thank the vacuum group of the Advanced Photon Source at Argonne National Laboratory for their work in this area.
Richard Bothell
Richard Bothell is former President of Atlas Technologies.
Jed Bothell
Jed Bothell is owner and President of Atlas Technologies and co-inventor of the Atlas Flange. Atlas Technologies supplies aluminum/stainless and copper/stainless bonded flanges.